As far as I can tell battery research seems to consist of mixing every single element with lithium, and seeing if it makes a battery.
Followed by advertising it and never releasing the new tech.
That’s because lithium is in the most electropositive group of elements and sodium/potassium are too reactive for current technology. Theoretically I think Na and K based batteries should perform better as they’re even more electropositive than Li.
(Forgive the spelling error in the picture but it was the simplest one I could find quickly)
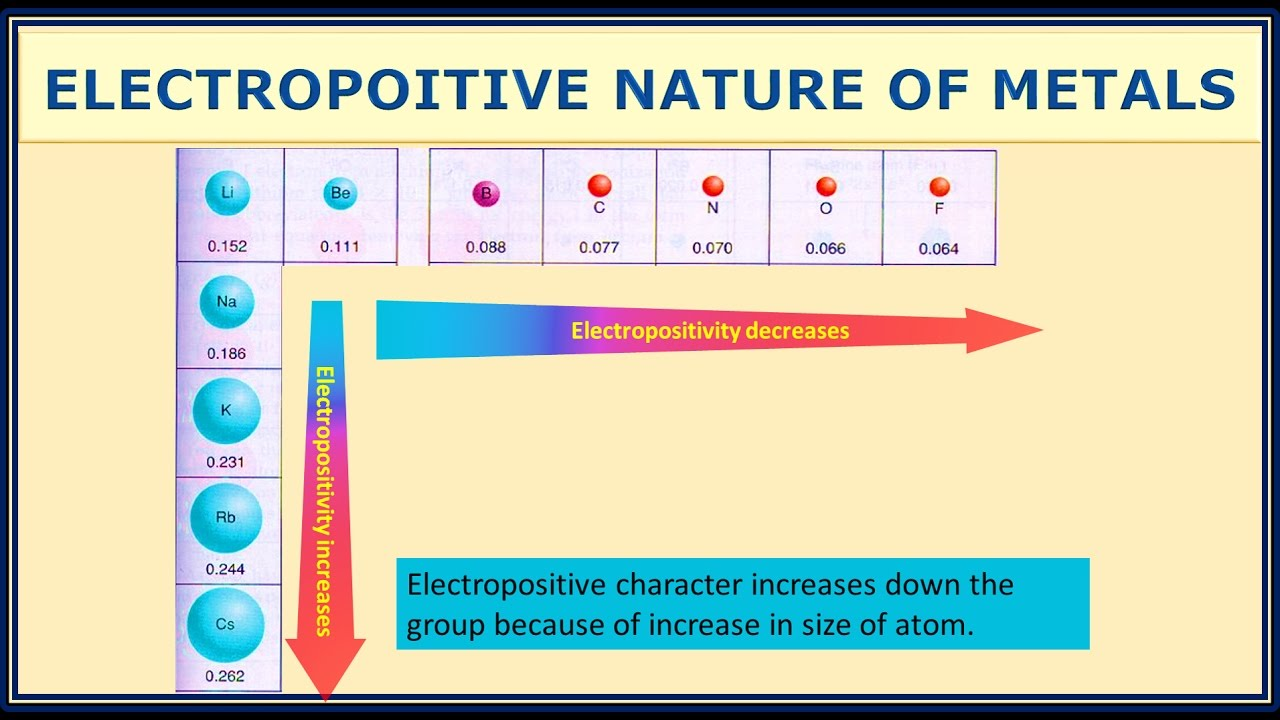
Na and K based batteries should perform better
What I’m hearing is throw some salt on a banana and power my phone for days.
I wasn’t very good at chemistry.
It’s the difference in electronegativity that makes the battery. That’s why you see lithium and oxygen a lot; lithium doesn’t want electrons, oxygen does want them. Sodium and potassium are very close in electronegativity so the salty banana battery wouldn’t be good.
I’m waiting for the cesium / fluorine battery, should theoretically be awesome. Or extremely explosive
That’s a much more serious and informative answer than I deserved.
Thank you for the explanation.
Gotta put my chemistry education to good use somehow, certainly not using it in the IT career I ended up getting in.
The other thing for lithium is that its light, VERY light, which of course is ideal for hand sets. Manufacturers love the light and slim designs even though consumers would prefer to have a handset that can go 7 days without a charge
This is more accurate than you would think. I’ve seen people synthesize a new inorganic compound, and is then more or less forced by supervisors to test it as an intercalation host for Li- or Na-ion batteries without really having thought through whether that makes sense at all.
Li is small, and as long as there is room for it (sites for it to sit when intercalated and paths to diffuse through the material), and there is some species that can accommodate the additional charge (as one Li+ is introduced into the material, there needs to be a charge compensation to maintain charge neutrality - typically this is a transition metal cation that is reduced from a higher oxidation state to a lower one). In that sense a lot of materials could serve as hosts, and depending on the intercalation potential, it could be used as a cathode (LiCoO2 for instance, where the intercalation potential vs. Li/Li+ is so high that it makes for a good cathode) or an anode (LTO for instance, where the intercalation potential vs. Li/Li+ is so low that it rather makes sense to pair it with a high potential cathode, and instead make for a more niche application where things such as safety is more coveted). That said, only three structure types have been widely used commercially as intercalation hosts for Li-ion batteries: layered rocksalt types (like LiCoO2 and its deriviates, NMC and NCA), spinels (LiMn2O4 or LTO) or olivines (LiFePO4, or LFP).
Li-S is not someone randomly mixing Li with some other elements though, it has been researched for a long time and is considered one of several “holy grails”
Xiaomi 11i Hypercharge can go from 0% to 100% in 15 minutes. And it’s a 2021 phone.
I dont give a shit fucking care untill i can buy it. Ive heard a million “this new battery tech will change the world” headlines.
New battery tech HAS changed the world though. We wouldn’t have modem EVs, smartphones, etc with the batteries of yesteryear.
Then get out of technology and start reading posts in buy it for life.
Even under rapid charging conditions with a full charge time of just 12 minutes, the battery achieved a high capacity of 705 mAh g⁻¹, which is a 1.6-fold improvement over conventional batteries. Furthermore, nitrogen doping on the carbon surface effectively suppressed lithium polysulfide migration, allowing the battery to retain 82% capacity even after 1,000 charge–discharge cycles, demonstrating excellent stability.
Assuming that this is scalable for production… Which is a big if for many of these “breakthroughs”, then this could replace current Lithium Ion batteries in most devices with a noticeable bump in capacity. Everything else is pretty par for the course though with current technologies.
The full charge time is meaningless without knowing what capacity they were working with. And a quick skim didn’t seem to have that in the article.
I’ve read news about better battery technology for YEARS, and then nothing. Repeat the cycle.
Let me know when it’s released to the public and actually usable.
Lots of small, incremental improvements. The news predictably is always promising a huge breakthrough
“We made a minor incremental improvement to our manufacturing process using existing technologies what will improve battery cycles by 1%! Amazing!”
“Fully charged in 12 minutes” is meaningless without a capacity.
I agree, the title is pretty useless.
Even something like “Fully charged a double A battery in 28 seconds” would’ve been useful/interesting.
Indeed. A modern Nissan Leaf with a 62 kWh battery can charge in a little over 11 minutes if you have a 2kV 160 amp line to toss into it. Because you know, it’s completely safe and cool to deal with those kinds of values for the average consumer.